As summer winds to a close, and fall classes loom closer and closer, we recognize that it will be difficult for us to post by our regular schedule (as in, a greater deviation than usual).
However, rest assured that updates from all subteams will still be completed on a regular basis! Right, guys?
:D Enjoy the rest of the summer, and see you in September!
-Mandy
Friday, August 21, 2009
Thursday, August 20, 2009
Labbing it up!
Hello virtual world of synthetic biologists, engineers and the rest of the cool kids floating around in cyber space!
Jamie is back with a short and sweet update from the lab side of things.
This past week Emily (with a little Vicki-probably about 0.1 of her to be exact) alongside with Kevin proceeded to test the reporter circuit with the mutant circuits. This involved a quick plasmid switch of the mutant Vicki, I mean OD47A into psB1AC3 simply due to antibiotic resistance and selection pressure. Overnights were grown and tested in the plate reader Synergy HT.
Jeremy and myself (Jeremy did most if not all of the pipetting; I might have plated one or two transformations here and there) just finished up the signalling pathway in pCS26. Sequencing results were analyzed with tools available over at ncbi.nlm.nih.gov (i.e. BLAST) and the signalling cascade is now officially completed with glycerol stocks, plasmid DNA and restreaks ready to go!
I (sort of) lied when I said the luxPQ-luxOU construct was done. Just a tad. Carol's project involving the synthetic sigma70 promoter is currently on hold as not only is Carol off in Vancouver on a well deserved holiday but new primers need to be made with the degenerate bases. We hope to resume progress on this after next week.
Meanwhile, I myself am fiddling around with mineral oil, plate reader, 96 well microplates,Vibrio harveyiSalmonella typhimurium, Escherichia coli, centrifuges in hopes of isolating AI-2. Please note this is all done without the handy dandiness of multi-channel pipettes or access to a VICTOR; nevertheless learning to pipette effectively and how to calibrate a different model of plate is useful and exciting!
Without further ado, on behalf of the lab team I will like to sign off (not permanently though ;) - can't get rid of us THAT easily) for the summer. Thank you everyone for following us throughout the sunny (and rainy) summer days and I look forward to blogging again once the school year rolls around. So until next next week a.k.a. September, see you later :D!
Jamie is back with a short and sweet update from the lab side of things.
This past week Emily (with a little Vicki-probably about 0.1 of her to be exact) alongside with Kevin proceeded to test the reporter circuit with the mutant circuits. This involved a quick plasmid switch of the mutant Vicki, I mean OD47A into psB1AC3 simply due to antibiotic resistance and selection pressure. Overnights were grown and tested in the plate reader Synergy HT.
Jeremy and myself (Jeremy did most if not all of the pipetting; I might have plated one or two transformations here and there) just finished up the signalling pathway in pCS26. Sequencing results were analyzed with tools available over at ncbi.nlm.nih.gov (i.e. BLAST) and the signalling cascade is now officially completed with glycerol stocks, plasmid DNA and restreaks ready to go!
I (sort of) lied when I said the luxPQ-luxOU construct was done. Just a tad. Carol's project involving the synthetic sigma70 promoter is currently on hold as not only is Carol off in Vancouver on a well deserved holiday but new primers need to be made with the degenerate bases. We hope to resume progress on this after next week.
Meanwhile, I myself am fiddling around with mineral oil, plate reader, 96 well microplates,Vibrio harveyiSalmonella typhimurium, Escherichia coli, centrifuges in hopes of isolating AI-2. Please note this is all done without the handy dandiness of multi-channel pipettes or access to a VICTOR; nevertheless learning to pipette effectively and how to calibrate a different model of plate is useful and exciting!
Without further ado, on behalf of the lab team I will like to sign off (not permanently though ;) - can't get rid of us THAT easily) for the summer. Thank you everyone for following us throughout the sunny (and rainy) summer days and I look forward to blogging again once the school year rolls around. So until next next week a.k.a. September, see you later :D!
FM 707.1 Marketing Update for the Week of August 17th 2009
Hello everyone,
My name is Fahd Mirza and you are reading my FM 707.1 marketing update for the week of August 17th. This week was rather slow for marketing because our marketing team was either busy with lab or ethics work. However we still managed to get a lot of stuff done for marketing,
1) Last Thursday, we were interviewed by the CJSW 90.9 Radio station. CJSW is the University of Calgary Community Radio station whose aim is to promote the activities of the University of Calgary students in Calgary and surrounding areas. We were interviewed by Joe Burima, who is the current program director for CJSW. On behalf of the University of Calgary iGEM team, we would like to sincerely thank CJSW for their support to iGEM and hope that they would continue to support our team in the future.
2) This week, Prima and I continued to contact Syn-Bio and Oil & Gas Companies for potential sponsorship/partnership. We hope to wrap up the sponsorship agenda by the end of August.
3) The biggest news of this week is that we have surpassed the amount of funds that we needed for this year. All the funds generated from now on will be contributed towards our next year’s iGEM fund for recruitment and other purposes.
4) We have also been working on our August newsletter which will be out soon.
WHAT’S NEXT?
We have also been working on our August newsletter which will be out soon. Also Prima has contacted the company that will be responsible for making our University of Calgary 2009 iGEM team shirts. We will also be working on attracting the media’s attention for our aGEM and iGEM jamborees.
My name is Fahd Mirza and you are reading my FM 707.1 marketing update for the week of August 17th. This week was rather slow for marketing because our marketing team was either busy with lab or ethics work. However we still managed to get a lot of stuff done for marketing,
1) Last Thursday, we were interviewed by the CJSW 90.9 Radio station. CJSW is the University of Calgary Community Radio station whose aim is to promote the activities of the University of Calgary students in Calgary and surrounding areas. We were interviewed by Joe Burima, who is the current program director for CJSW. On behalf of the University of Calgary iGEM team, we would like to sincerely thank CJSW for their support to iGEM and hope that they would continue to support our team in the future.
2) This week, Prima and I continued to contact Syn-Bio and Oil & Gas Companies for potential sponsorship/partnership. We hope to wrap up the sponsorship agenda by the end of August.
3) The biggest news of this week is that we have surpassed the amount of funds that we needed for this year. All the funds generated from now on will be contributed towards our next year’s iGEM fund for recruitment and other purposes.
4) We have also been working on our August newsletter which will be out soon.
WHAT’S NEXT?
We have also been working on our August newsletter which will be out soon. Also Prima has contacted the company that will be responsible for making our University of Calgary 2009 iGEM team shirts. We will also be working on attracting the media’s attention for our aGEM and iGEM jamborees.
Tuesday, August 18, 2009
Letter from one island to another
Dear Mandy,
Due to finals early this week and you having left for Hawaii, second life team members have been sparse. I wanted to stay in touch and keep you up to date somehow.
As you are aware, since scripting and equipment in the lab has been completed, last week we began the base of the Biobrick spiral where some of the fundamentals of molecular biology, including the central dogma will be located. You will be happy to know I have completed the DNA replication animation that avatars will be able to click on in order to learn what is required to happen for successful replication as well as a initiation point/button to begin the activity as well as reset it. All that is left is make it user friendly since everything that can be touched will perform some action and these actions just need to be explained and secured so that order is important. This may also be done with the help of a notecard that has been made. The only reason this went so slowly is the fact that I am attempting to complete wiki notebook updates in parallel and believe me, it has been a painful experience decrypting the notes that I have. Now, transcription and translation will have to be added and will most likely be tackled by either you or myself in the near future. I have started the initial framework for transcription, but most likely will not be able to complete it before this week is finished.

As I understand it, the biobrick simulator is basically completed and now the levels have to be organized and the textures for the buttons of the biobrick simulator interface. Thank you for completing the buttons Patrick required for the HUD he has made. They look great and I’m sure he really appreciates it.
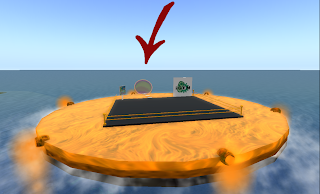
I see the area avatars first enter the island has been expanded by you and Stefan and now includes a map (including teleportation areas) and directive tubing into synthetic kingdom. To ignore the big red X’s and arrows would have to be intentional and pathway stones litter the landscape throughout. Stefan has been working on his eukaryotic cell and finishing up his disease hunting bacteria within it. Also, I would like to thank you for allocating an area for previous iGEM projects and I see that the logo now decorates the outer walls of the virtual lab. A section of the island will now be filled with worthy iGEM projects, which may be done in the fall with enough linden dollars.

Hoping you are able to locate a bobtail squid,
Katie
Due to finals early this week and you having left for Hawaii, second life team members have been sparse. I wanted to stay in touch and keep you up to date somehow.
As you are aware, since scripting and equipment in the lab has been completed, last week we began the base of the Biobrick spiral where some of the fundamentals of molecular biology, including the central dogma will be located. You will be happy to know I have completed the DNA replication animation that avatars will be able to click on in order to learn what is required to happen for successful replication as well as a initiation point/button to begin the activity as well as reset it. All that is left is make it user friendly since everything that can be touched will perform some action and these actions just need to be explained and secured so that order is important. This may also be done with the help of a notecard that has been made. The only reason this went so slowly is the fact that I am attempting to complete wiki notebook updates in parallel and believe me, it has been a painful experience decrypting the notes that I have. Now, transcription and translation will have to be added and will most likely be tackled by either you or myself in the near future. I have started the initial framework for transcription, but most likely will not be able to complete it before this week is finished.

As I understand it, the biobrick simulator is basically completed and now the levels have to be organized and the textures for the buttons of the biobrick simulator interface. Thank you for completing the buttons Patrick required for the HUD he has made. They look great and I’m sure he really appreciates it.

I see the area avatars first enter the island has been expanded by you and Stefan and now includes a map (including teleportation areas) and directive tubing into synthetic kingdom. To ignore the big red X’s and arrows would have to be intentional and pathway stones litter the landscape throughout. Stefan has been working on his eukaryotic cell and finishing up his disease hunting bacteria within it. Also, I would like to thank you for allocating an area for previous iGEM projects and I see that the logo now decorates the outer walls of the virtual lab. A section of the island will now be filled with worthy iGEM projects, which may be done in the fall with enough linden dollars.

Hoping you are able to locate a bobtail squid,
Katie
Monday, August 17, 2009
Sensitivity analysis: Models... have feelings too!
Vicki and Chinee has put quite an amout of time into tracking down those sensitive ones in our system. Vicki is currently working with the Sensitivity analysis tool in Matlab commandline in order to find the sensitive parameters that influence the GFP output the most. The following graph is the result her sensitivity analysis model has generated so far: (click to enlarge)

The closer the graph gets to 0, the lower the influence this parameter has on the GFP output, and vice versa. According to this graph, the kForward (reaction rates)values 1, 3, and 4 seems the most influential parameters relative to 2 and 5; thus these parameters will be looked at further.
From these results, Vicki performed parameter optimization on them. In parameter optimization function, she was able to play with the individual parameter values in order to come up with a best fit line model to the predicted levels of GFP output. The following is the graphical result of the above function.

Here, the circles represent the made up predicted data, and the colored lines represent each optimized parameters. The optimized rate constants for kForward1, 3, and 4 fit nicely to the pattern of the predicted data; however, the optimized rate constants for kForward 2 and 5 do not, meaning that these parameters do not play a big role in determining the GFP output values.
From these two functions, Vicki would be able to, once we get some lab results, optimize our significant rate constants to fit the behavior of our AI-2 system.
Along with Vicki, Chinee was also working on the parameter sensitivity; however, with a different tool. In Simbiology, Chinee was able to outline the key difference between the initial amount and the reaction rates of the parameter. The following is the graph when all the parameters have a reaction rate value of 1:

The following is the graph when all the parameters have a reaction rate value of 10:

The only difference between the above two graphs are that the lines in the second graph are much more steep than the lines in the first graph. This means that the reaction rate only influences how immediate the reactions happen.
And finally, the graph when all the parameters have an initial amount of 10:

The above graph is very different from the ones above. From this, one can see how initial amounts of parameter play a much bigger role than the rate constants of each parameters.
I, Kevin, and Carol attempted to fix some biological misunderstandings within the reactions, and produce a graph that displays the pattern of each parameters. The following is the graph:

The patterns observed are reasonable and match what was expected. The lab data is needed to get an accurate model of our system; however, from it we can still test the sensitivity of each parameters by changing them one by one and simulating the results.
We hope to get some lab data by this week.

The closer the graph gets to 0, the lower the influence this parameter has on the GFP output, and vice versa. According to this graph, the kForward (reaction rates)values 1, 3, and 4 seems the most influential parameters relative to 2 and 5; thus these parameters will be looked at further.
From these results, Vicki performed parameter optimization on them. In parameter optimization function, she was able to play with the individual parameter values in order to come up with a best fit line model to the predicted levels of GFP output. The following is the graphical result of the above function.

Here, the circles represent the made up predicted data, and the colored lines represent each optimized parameters. The optimized rate constants for kForward1, 3, and 4 fit nicely to the pattern of the predicted data; however, the optimized rate constants for kForward 2 and 5 do not, meaning that these parameters do not play a big role in determining the GFP output values.
From these two functions, Vicki would be able to, once we get some lab results, optimize our significant rate constants to fit the behavior of our AI-2 system.
Along with Vicki, Chinee was also working on the parameter sensitivity; however, with a different tool. In Simbiology, Chinee was able to outline the key difference between the initial amount and the reaction rates of the parameter. The following is the graph when all the parameters have a reaction rate value of 1:

The following is the graph when all the parameters have a reaction rate value of 10:

The only difference between the above two graphs are that the lines in the second graph are much more steep than the lines in the first graph. This means that the reaction rate only influences how immediate the reactions happen.
And finally, the graph when all the parameters have an initial amount of 10:

The above graph is very different from the ones above. From this, one can see how initial amounts of parameter play a much bigger role than the rate constants of each parameters.
I, Kevin, and Carol attempted to fix some biological misunderstandings within the reactions, and produce a graph that displays the pattern of each parameters. The following is the graph:

The patterns observed are reasonable and match what was expected. The lab data is needed to get an accurate model of our system; however, from it we can still test the sensitivity of each parameters by changing them one by one and simulating the results.
We hope to get some lab data by this week.
Thursday, August 13, 2009
FM 707.1 Marketing Update for the Weeks of August 3rd 2009 and August 10th 2009
Hi everyone,
My name is Fahd Mirza and you are reading the FM 707.1 Marketing Update for the week of August 3rd 2009 and August 10th 2009. These past two weeks have been intensely busy and highly productive for our marketing team.
Last week, we had organized a massive bake sale having more than 400 baked items to sell. This event managed to raise a net profit of $500.04 Cdn Dollars. All the funds would be directed towards our research project. We had also managed to get sponsorships from Corning Life Sciences (Thanks to Prima Moinul) and VWR Scientific (Courtesy Fahd Mirza) last week. Our July newsletter was also ready to be sent out to potential/current/future sponsors and to media organizations who gave us media coverage. I want to thank Jamie Feng and Prima Moinul for doing a marvelous job on the newsletter.
Nexen Inc. was declared our Sponsor of the Month for the month of August. It is with Nexen Inc.’s generous donation that iGEM Calgary would be able to achieve its goals.
This week, our iGEM Calgary team toured the Oil Sands in Fort McMurray courtesy of Andrew Hessel and OSLI (Oil Sands Leadership Initiative). The tour was a great learning experience and an exciting opportunity for iGEM Calgary team to work on future potential synthetic biology projects for iGEM. It was also an opportunity for our marketing team to form important partnerships with the Oil and Gas Development companies.
We are still working on getting more sponsorship for our research project and I hope that we are successful in this endeavor. Within the next couple of weeks our marketing team will be working on our August newsletter and getting media coverage for our project, the All Alberta iGEM Jamboree (also called aGEM) and the iGEM competition itself at MIT.
My name is Fahd Mirza and you are reading the FM 707.1 Marketing Update for the week of August 3rd 2009 and August 10th 2009. These past two weeks have been intensely busy and highly productive for our marketing team.
Last week, we had organized a massive bake sale having more than 400 baked items to sell. This event managed to raise a net profit of $500.04 Cdn Dollars. All the funds would be directed towards our research project. We had also managed to get sponsorships from Corning Life Sciences (Thanks to Prima Moinul) and VWR Scientific (Courtesy Fahd Mirza) last week. Our July newsletter was also ready to be sent out to potential/current/future sponsors and to media organizations who gave us media coverage. I want to thank Jamie Feng and Prima Moinul for doing a marvelous job on the newsletter.
Nexen Inc. was declared our Sponsor of the Month for the month of August. It is with Nexen Inc.’s generous donation that iGEM Calgary would be able to achieve its goals.
This week, our iGEM Calgary team toured the Oil Sands in Fort McMurray courtesy of Andrew Hessel and OSLI (Oil Sands Leadership Initiative). The tour was a great learning experience and an exciting opportunity for iGEM Calgary team to work on future potential synthetic biology projects for iGEM. It was also an opportunity for our marketing team to form important partnerships with the Oil and Gas Development companies.
We are still working on getting more sponsorship for our research project and I hope that we are successful in this endeavor. Within the next couple of weeks our marketing team will be working on our August newsletter and getting media coverage for our project, the All Alberta iGEM Jamboree (also called aGEM) and the iGEM competition itself at MIT.
CLOSE UP of iGEM Calgary's LAB
Hey guys! Summer is coming to a close pretty quick here, and so is iGEM! We are working tremendously hard to try and bring our project to completion! Here’s where we stand as of today (August 12, 2009):
Signaling Circuit
LuxPQ-B0015-R0040-LuxOU-B0015 has been successfully verified in psB1AC3. But that’s old news. We have recently cloned this construct into the pCS26 vector, whose cloning site originally had LuxCDABE flanked by NotI sites. We cut our signaling construct with NotI enzyme, but this does not guarantee that our construct was cloned in the appropriate direction. We transformed the product into TOP10 and XL Gold competent cells, and grew them on Kanamycin plates (pCS26 has resistance for this antibiotic). We have isolated plasmid from eight colonies, and we will verify whether the direction of our construct is correct with a primer that anneals just outside the cloning site on the supposed LuxPQ side. This primer will be paired with a LuxPQ Reverse primer. If we see a PCR product of just over 4kb, we have cloned the construct in the right direction, if we get smearing, however, we have not cloned the product in the right direction.
Moving forward, we require the sigma 70 promoter library that will control the expression levels of LuxPQ in our system. We have had trouble cloning this into the pCS26 vector, and will be trying this again from the start.
Reporter Circuit and Mutants
Pqrr4-B0034-GFP has been successfully verified in psB2K3. Both mutants have been verified in the following constructs in psB1AC3: R0040-B0034-LuxO D47A-B0015 and R0040-B0034-LuxO D47E-B0015. The Pqrr4-B0034-GFP is currently in TOP10 cells, and being made competent once again in order to allow the subsequent transformation of the mutants into the cells. The purpose here is to test the mutant circuits to see if they are working.
Acquiring AI-2
The purpose of constructing all these circuits is to make an AI-2 signaling system. So what else do we need? AI-2! We are currently isolating AI-2 from a species of Salmonella by centrifuging and taking the supernatant that should contain the AI-2. Many controls will be used in order to test whether AI-2 is actually present in the supernatant, namely using Vibrio Harveyi as a response which should glow in the presence of AI-2 because it naturally has the AI-2 signaling system. Once verified that we have AI-2, it can then be used to see if our system responds to AI-2.
Signaling Circuit
LuxPQ-B0015-R0040-LuxOU-B0015 has been successfully verified in psB1AC3. But that’s old news. We have recently cloned this construct into the pCS26 vector, whose cloning site originally had LuxCDABE flanked by NotI sites. We cut our signaling construct with NotI enzyme, but this does not guarantee that our construct was cloned in the appropriate direction. We transformed the product into TOP10 and XL Gold competent cells, and grew them on Kanamycin plates (pCS26 has resistance for this antibiotic). We have isolated plasmid from eight colonies, and we will verify whether the direction of our construct is correct with a primer that anneals just outside the cloning site on the supposed LuxPQ side. This primer will be paired with a LuxPQ Reverse primer. If we see a PCR product of just over 4kb, we have cloned the construct in the right direction, if we get smearing, however, we have not cloned the product in the right direction.
Moving forward, we require the sigma 70 promoter library that will control the expression levels of LuxPQ in our system. We have had trouble cloning this into the pCS26 vector, and will be trying this again from the start.
Reporter Circuit and Mutants
Pqrr4-B0034-GFP has been successfully verified in psB2K3. Both mutants have been verified in the following constructs in psB1AC3: R0040-B0034-LuxO D47A-B0015 and R0040-B0034-LuxO D47E-B0015. The Pqrr4-B0034-GFP is currently in TOP10 cells, and being made competent once again in order to allow the subsequent transformation of the mutants into the cells. The purpose here is to test the mutant circuits to see if they are working.
Acquiring AI-2
The purpose of constructing all these circuits is to make an AI-2 signaling system. So what else do we need? AI-2! We are currently isolating AI-2 from a species of Salmonella by centrifuging and taking the supernatant that should contain the AI-2. Many controls will be used in order to test whether AI-2 is actually present in the supernatant, namely using Vibrio Harveyi as a response which should glow in the presence of AI-2 because it naturally has the AI-2 signaling system. Once verified that we have AI-2, it can then be used to see if our system responds to AI-2.
Wednesday, August 12, 2009
Crunch Time in Second Life
Hello again! This week has been another busy week for us Second Lifers. We are wrapping up a lot of our work for the summer. Patrick's update about the biobricker from last week was awesome, and this week I'll be describing what we've been up to in the Synthetic Kingdom and the Labs.
In the Synthetic Kingdom (where we show potential applications of synthetic biology), we are completing the pathway through the levels, and the descriptions and instructions for the stations. We will also be completing descriptions of free moving bacteria that can be interacted with, which visitors can try to look for them while following the path, creating a dynamic environment that still provides guidance for users. This area will also be expanded to include descriptions of past iGEM projects in an "iGEM Hall of Fame". The pathway through the kingdom is complete, as well as the individual stationed activities and the drop in point. Now we are just building on it to make it clear what sort of things we want users to learn as they travel through the kingdom.
All of the lab scripts are complete, and all of the lab activity parts have been duplicated and modified for the second lab. We are still working on the instructions and descriptions for each of these activities. Now that the lab missions are fully functional, we've also created prizes to be given for successful completion of the lab , as an incentive. As the lab missions get successively more difficult, the awesomeness of the prizes increases. :)
Since the lab component is mainly complete, we have begun working on base of the biobricker helix, where we will be putting up exhibits and other objects that will explain some basics: what are genes, how gene expression can be modified, replication, transcription, translation, and some important parts of iGEM (the registry, how biobricking works, etc.).
We're getting nearer to the end of summer, so now we're trying to complete as much as we can in hopes of having most (if not all) of the island ready for people to explore.
In the Synthetic Kingdom (where we show potential applications of synthetic biology), we are completing the pathway through the levels, and the descriptions and instructions for the stations. We will also be completing descriptions of free moving bacteria that can be interacted with, which visitors can try to look for them while following the path, creating a dynamic environment that still provides guidance for users. This area will also be expanded to include descriptions of past iGEM projects in an "iGEM Hall of Fame". The pathway through the kingdom is complete, as well as the individual stationed activities and the drop in point. Now we are just building on it to make it clear what sort of things we want users to learn as they travel through the kingdom.
All of the lab scripts are complete, and all of the lab activity parts have been duplicated and modified for the second lab. We are still working on the instructions and descriptions for each of these activities. Now that the lab missions are fully functional, we've also created prizes to be given for successful completion of the lab , as an incentive. As the lab missions get successively more difficult, the awesomeness of the prizes increases. :)
Since the lab component is mainly complete, we have begun working on base of the biobricker helix, where we will be putting up exhibits and other objects that will explain some basics: what are genes, how gene expression can be modified, replication, transcription, translation, and some important parts of iGEM (the registry, how biobricking works, etc.).
We're getting nearer to the end of summer, so now we're trying to complete as much as we can in hopes of having most (if not all) of the island ready for people to explore.
Saturday, August 8, 2009
Modeling (Membrane Computing) Update
Hi Everyone!
This will be our first update on Membrane Computing (MC) and agent-based approach to model our complex biological system.
In regards to modeling of Autoinducer-II Quorum Signaling, we have been able to successfully complete our implementation of this signaling cascade. Monitoring each cell as an agent, we are able to monitor the system at both, cell level and population level.
Currently our simulation uses a well known algorithm called Gillespie's Algorithm. In simple words, the Gillespie algorithm keeps stochasticity (randomness) in our simulation. Why is it important to have this randomness in our model? Because this will push our model one step closer to actual biological system. Because cells don't interact with each other or their environment at discrete steps. As an example consider protein-protein interactions versus cell division. These two interactions have quite different time scales. Our implementation of Gillespie Algorithm takes care of accounting for such cases.
At this stage our model is able to produce concentration graphs and allows user to review how the system behaves at the cell level and also at colony level. We are working on implementing powerful visualizations and a user-friendly interface for our model.
We are also working on addition of new updating scheme for cells during simulation. Currently our algorithm updates state of each cell at single steps. It is worth mentioning that because of the way our Gillespie Algorithm is setup, time delays between each step can be as short as 0.0001 second (considered instantaneous) or a given step could take as long as one minute. This will compensate for different interactions and the time it takes for each interaction to complete. The second scheme which we are working now to complete hopefully before aGEM is where all cells in the system are updated at the same time. This allows cells to be in sync when applying interactions. With single-step updating scheme, as the number of cells increase over time, it takes longer and longer for cells to update their state based on changes in their environment. This side effect of non-synchronous updating scheme only kicks in when we have high population of cells. Since we have introduced cell division to our simulation where our system could start with one cell and end up with 300, a synchronous updating system could take us one step closer to how the actual biological system works.
We will post updates at least once a week on our current progress; We also hope to have some screen shots on our visualizations and interface in near future.
Thanks for following our progress :)
This will be our first update on Membrane Computing (MC) and agent-based approach to model our complex biological system.
In regards to modeling of Autoinducer-II Quorum Signaling, we have been able to successfully complete our implementation of this signaling cascade. Monitoring each cell as an agent, we are able to monitor the system at both, cell level and population level.
Currently our simulation uses a well known algorithm called Gillespie's Algorithm. In simple words, the Gillespie algorithm keeps stochasticity (randomness) in our simulation. Why is it important to have this randomness in our model? Because this will push our model one step closer to actual biological system. Because cells don't interact with each other or their environment at discrete steps. As an example consider protein-protein interactions versus cell division. These two interactions have quite different time scales. Our implementation of Gillespie Algorithm takes care of accounting for such cases.
At this stage our model is able to produce concentration graphs and allows user to review how the system behaves at the cell level and also at colony level. We are working on implementing powerful visualizations and a user-friendly interface for our model.
We are also working on addition of new updating scheme for cells during simulation. Currently our algorithm updates state of each cell at single steps. It is worth mentioning that because of the way our Gillespie Algorithm is setup, time delays between each step can be as short as 0.0001 second (considered instantaneous) or a given step could take as long as one minute. This will compensate for different interactions and the time it takes for each interaction to complete. The second scheme which we are working now to complete hopefully before aGEM is where all cells in the system are updated at the same time. This allows cells to be in sync when applying interactions. With single-step updating scheme, as the number of cells increase over time, it takes longer and longer for cells to update their state based on changes in their environment. This side effect of non-synchronous updating scheme only kicks in when we have high population of cells. Since we have introduced cell division to our simulation where our system could start with one cell and end up with 300, a synchronous updating system could take us one step closer to how the actual biological system works.
We will post updates at least once a week on our current progress; We also hope to have some screen shots on our visualizations and interface in near future.
Thanks for following our progress :)
Wednesday, August 5, 2009
Second Life: Belated Update!
Hello again,
This will be a shorter update than the last few weeks, mostly because the SL team is hard at work. Our iGEM season ends alarmingly soon, on the 21st of August, so we're in crunch mode now to get the sim polished and ready for public consumption.
I will tell you what I've been up to toward the end of last week and the beginning of this one: the Biobrick builder (aka the Biobricker). This is one of the most important features of the Biobrick Simulator in SL, because it turns the very static devices seen in the video last week into plastic and redesignable systems. The interface for the Biobricker is finished, which enables the user to piece various promoters, coding sequences, terminators, and other Biobrick parts together. You can build a device of any length, insert and delete parts anywhere in the Biobrick too. The system isn't only critical for the final package, it will be very useful for testing the other new features that are yet to come. Clicking around the interface already feels nice and responsive, and very powerful!
The system is designed to be expandable, so while the initial selection of parts will be small it will be easy for myself or another intrepid SL coder to add additional elements. Did I mention that all of the components we're designing this summer will be made available for all to use in Second Life? We saw no point in restricting the use of the work we've done this summer, and I'd love to see what people can come up with.
The interface was finished up last week, but I have just finished the Linden Scripting Language code (that's Second Life's built in scripting language) to accomplish the construction of these Biobricks in world today. The Biobricker needs so pass a few more trace-throughs and a lot of testing before I certify this part of the project complete though.
A simplified view of what's going on behind the scenes, is that after using the Biobricker's interface to design the device you want, the interface object directs the assembly of a number of Biobrick objects in the real world. It creates each Biobrick part, gives it a name and position within the completed device, and orders all the DNA to link together into the final device. Guaranteeing that each part gets the information it needs is harder than it sounds though, especially given the nature of communications in Second Life! It's impossible to guarantee messages will arrive in the order they were sent, or that they will even arrive at all, so the construction system has a lot of fail-safes to ensure that your device comes out right every time.
That's all for this week, stay tuned!
This will be a shorter update than the last few weeks, mostly because the SL team is hard at work. Our iGEM season ends alarmingly soon, on the 21st of August, so we're in crunch mode now to get the sim polished and ready for public consumption.
I will tell you what I've been up to toward the end of last week and the beginning of this one: the Biobrick builder (aka the Biobricker). This is one of the most important features of the Biobrick Simulator in SL, because it turns the very static devices seen in the video last week into plastic and redesignable systems. The interface for the Biobricker is finished, which enables the user to piece various promoters, coding sequences, terminators, and other Biobrick parts together. You can build a device of any length, insert and delete parts anywhere in the Biobrick too. The system isn't only critical for the final package, it will be very useful for testing the other new features that are yet to come. Clicking around the interface already feels nice and responsive, and very powerful!
The system is designed to be expandable, so while the initial selection of parts will be small it will be easy for myself or another intrepid SL coder to add additional elements. Did I mention that all of the components we're designing this summer will be made available for all to use in Second Life? We saw no point in restricting the use of the work we've done this summer, and I'd love to see what people can come up with.
The interface was finished up last week, but I have just finished the Linden Scripting Language code (that's Second Life's built in scripting language) to accomplish the construction of these Biobricks in world today. The Biobricker needs so pass a few more trace-throughs and a lot of testing before I certify this part of the project complete though.
A simplified view of what's going on behind the scenes, is that after using the Biobricker's interface to design the device you want, the interface object directs the assembly of a number of Biobrick objects in the real world. It creates each Biobrick part, gives it a name and position within the completed device, and orders all the DNA to link together into the final device. Guaranteeing that each part gets the information it needs is harder than it sounds though, especially given the nature of communications in Second Life! It's impossible to guarantee messages will arrive in the order they were sent, or that they will even arrive at all, so the construction system has a lot of fail-safes to ensure that your device comes out right every time.
That's all for this week, stay tuned!
Lab: Some Progress Finally!!!
Hi Everyone! Thanks for reading our lab blog once again! Just a quick lab update for all you readers this week. Emily has finally completed her mutant circuit for
luxOD47E. Congrats to her! Jeremy and Jamie are currently working on several things right now. First, they finally performed a plasmid switch from pSB1AK3 to pSB1AC3 for luxPQOU. Now that they have successfully switched plasmids, they will be constructing luxPQOU into pCS26 (surette vector) by cutting with NotI enzyme. Finally, they are working on verifying cllamda. Unfortunately, after many enzyme digestions, they are unable to verify that the sequence is in the vector. They are currently trying again and hopefully will get results later this week. Kevin is working on the reporter circuit and he is verifying that circuit today with restriction enzymes. He is going to look into how to test the mutant circuits this week as well. Finally I am still stuck with the sigma 70 promoter. Unfortunately, I am still unable to get any colonies. We are currently looking at other ways to optimize the results. Hopefully, I'll have better results to report next week! Thanks for reading!
luxOD47E. Congrats to her! Jeremy and Jamie are currently working on several things right now. First, they finally performed a plasmid switch from pSB1AK3 to pSB1AC3 for luxPQOU. Now that they have successfully switched plasmids, they will be constructing luxPQOU into pCS26 (surette vector) by cutting with NotI enzyme. Finally, they are working on verifying cllamda. Unfortunately, after many enzyme digestions, they are unable to verify that the sequence is in the vector. They are currently trying again and hopefully will get results later this week. Kevin is working on the reporter circuit and he is verifying that circuit today with restriction enzymes. He is going to look into how to test the mutant circuits this week as well. Finally I am still stuck with the sigma 70 promoter. Unfortunately, I am still unable to get any colonies. We are currently looking at other ways to optimize the results. Hopefully, I'll have better results to report next week! Thanks for reading!
Monday, August 3, 2009
Modelling: Collaborative Work
Hi Everyone! Not much to update for modelling this week. We had a big meeting with all the teams on friday, and the two modelling teams (Membrane Computing and Mathematical Modelling) will meet every tuesday and thursday for meetings. This will give us time to draw parallel between the two types of modelling. We have also discussed about how to seperate all the experiments to characterize our signalling system. All this detail will be touched tomorrow at the meeting. Other than that, not much to update. Hopefully next week, we have more to add! Peace :)
Subscribe to:
Posts (Atom)




